by-bottle quality control reagent testing|URI MLS: Urinalysis Ch. 5 (Chemical Examination) Flashcards : distribution Study with Quizlet and memorize flashcards containing terms like Proper care of reagent strips includes all of the following except: A. Checking the expiration date B. Storing in a clear container C. Preventing exposure to toxic fumes D. Storing with a desiccant, Quality control on reagent strips must be performed whenever a/an: A. Abnormal result is obtained B. Different person . Jungle Delight - real money online casino game for free (demo). See also another gamble and cash games like slots and roulette games in our catalog. Olá, Bem-vindo! Inscrever-se ou Iniciar sessão. Obter suporte. PT (Português) BR (Português) ES (Español) CL (Español)
{plog:ftitle_list}
webBesides, online is a must for these types of games, so the real ones interest (league racers and esports people) have already bought/been gifted the game. Not saying none of us aren't interested in F1 2023 getting cracked, but the few people with skills to do so, have bigger releases to deal with. 1. bludowski69.
Urinalysis quality control at the point
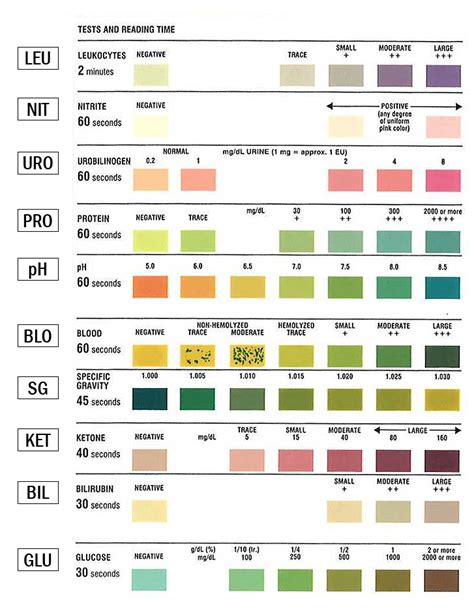
Urinalysis dipsticks contain discrete reagent pads to semi-quantitatively test for the presence of bilirubin, blood, creatinine, glucose, .List five quality-control procedures routinely performed with reagent strip testing.

london covid test package
Study with Quizlet and memorize flashcards containing terms like Proper care of reagent strips includes all of the following except: A. Checking the expiration date B. Storing in a clear container C. Preventing exposure to toxic fumes D. Storing with a desiccant, Quality control on reagent strips must be performed whenever a/an: A. Abnormal result is obtained B. Different person .Quality control of reagent strips is performed: A. . A. Removing the desiccant from the bottle B. Storing in an opaque bottle C. Storing at room temperature D. Resealing the bottle after removing a strip. . The principle of the reagent strip test for pH is the: Should be recollected. A urine specimen with a pH of 9.0: D. In the laboratory, . Quality control testing was performed daily for 15 days. . Internal Quality Control Data of Urine Reagent Strip Tests and . Derivation of Control Rules Based on Sigma Metrics. Ann Lab Med 2021 .
QUALITY CONTROL For best result, performance of reagent strips should be confirmed by testing known negative and positive specimens or controls whenever a new test is performed of whenever a new bottle is first opened. Each laboratory should establish its own goals for adequate standards of performance, and should question handling and testingEquivalent Quality Control (EQC) Aka: QC performed once every 30 days and any frequency other than each day of patient testing COMING 1/1/2016 Individualized Quality Control Plan (IQCP) Aka: QC frequency will need to be determined based on IQCP Risk Assessment 4
Quality Control; True or False: Urine chemical reagent strip readers can utilize any manufacturer's dipsticks. When an automated or semiautomated method is used to read urine chemical reagent strips, quality control testing must be performed: Summary Tables; Test Principles, Reactions, and Interfering Substances; References; ReferencesStudy with Quizlet and memorize flashcards containing terms like 1. The integrity of reagent strips is best assessed by: A. Using automated instrumentation B. Running control materials C. Keeping the lid tightly closed on the bottle D. Checking for a color change on the pads, 2. While performing UAs, the technician ran controls and began testing the specimens. He ran out of .Patient Samples require 6 drops of Extraction Reagent. Wrong . 1 . Correct x6 Hold Extraction Reagent bottle vertically. Hovering 1/2 inch above the . TOP HOLE, slowly add . 6 DROPS. to the . TOP HOLE
Test kit reagents/cards must be at room temperature before use. If . Quality control testing should also be performed after receiving new shipments of test kits. Training should include successfully testing . reagent, hold the bottle vertically (not at an angle) ½ inch above the card and slowly add 6 .3. Test the control solutions (Level 1 and Level 2) by squeezing the control bottle and drawing the control solution across all of the reagent pads, thoroughly saturating each pad. Do not aspirate excess control back into the bottle. Drain excess from dipstick. Time appropriately.1. Test open bottles of reagent strips with known positive and negative controls every 24 hours. 2. Resolve control results that are out of range by further testing. 3. Test reagent used in backup tests with positive and negative controls. 4. Perform positive and negative controls on new reagents and newly opened bottle of reagent strips. 5.Quanti-Cult Procedure a) Good laboratory practices will be used for this procedure. b) Pre-heat incubator to bring temperature up to 35ºC. c) Pre-warm rehydration fluid vials to 35º-37ºC. Use blue autoclavable foam vial holder to hold vials. d) Discard blue cap from rehydration fluid vial. e) Remove organism vial from pouch (vial with colorless cap). f) Transfer colorless cap onto pre .
Study with Quizlet and memorize flashcards containing terms like Using quality control materials, one should check reagent strip performance 1. at least once daily 2. when a new bottle of strips or tablets is opened 3. when a new lot number of strips or tablets is placed into use 4. once each shift by each laboratorian performing urinalysis testing, Urine pH can be modified .QUALITY CONTROL: CLIA Waived laboratories (U.S. only):Test negative and positive con-trols whenever a new reagent bottle is opened.Liquid, ready-to-use con-trols should be used. Water should not be used as a negative control. . The reagent test areas on CLINITEK Microalbumin Reagent Strips areWhen an automated or semi-automated method is used to read urine reagent strips, quality control testing must be performed at least: Every day of patient testing and when a new bottle is opened. . All of the following are true in .Study with Quizlet and memorize flashcards containing terms like if you have to open a new bottle of reagent strips or a new test kit during the day, you might need to ., With strict regulations regarding quality control and quality assurance, you are required to keep a log book to record all specimens and the results of the tests per- formed. The log should include:, The .
Quality control (QC) laboratories in the pharmaceutical industry depend on the precise use of laboratory reagents (solids, liquids, gases, and solutions) to carry out accurate analytical testing, making the establishment of expiry dates for both commercial and in-house prepared reagents a critical aspect of maintaining testing integrity and consistency. Internal Quality Control Data of Urine Reagent Strip Tests and Derivation of Control Rules Based on Sigma Metrics. Haeil Park, M.D., Ph.D. and Younsuk Ko, M.T. Author . Urine reagent strip test (URST) results are semi-quantitative; therefore, the precision of URSTs is evaluated as the proportion of categorical results from repeated .Quality control (QC) measures help ensure a standard level of testing quality, detect immediate errors and monitor the . laboratories in preserving reagents while maintaining quality testing only during the COVID-19 public health emergency (see . CMS FAQ #15 [12-17-2020]).
Quality control of reagent strips is performed: a) using positive and negative controls b) . storing at room temperature d) resealing the bottle after removing the strip. a) removing the desiccant from the bottle. . The primary chemical on the reagent strip in the Micral Test for micro albumin binds to: a) protein b) antihuman albumin .if you have to open a new bottle of reagent strips or a new test kit during the day, you might need to: . change the code to agree with what is printed on the bottle, and repeat the quality controls. with strict regulations regarding quality control and quality assurance, you are required to keep a log book to record all specimens and the .Quality Control Each bottle of Multistix 10 SG reagent strips will have QC performed using known positive and negative controls. This function will be carried out by testing personnel. Please notify the central laboratory when opening a new bottle. Records of quality control will be kept in the central laboratory. 2. Reagents. 5% Alpha-naphthol Solution (Barritt’s Reagent A) and 40% KOH or NaOH solution (Barritt’s Reagent B) are required. Preparation of Barritt’s Reagent A. Dissolve 5 grams of α-naphthol reagent in 100 mL of 95% ethanol. The reagent can be stored for up to 3 weeks in a dark place at 4 to 8°C. Preparation of Barritt’s Reagent B
positive and negative controls to ensure that test reagents are working and that the test is correct. BinaxNOW™ COVID-19 Ag Card kits contain a Positive Control Swab and Blank Sterile Swabs that can be used as a Negative Control Swab – These swabs will monitor the entire assay. Quality control testing must be performed: 1. A reagent control is a reagent made to the same formulation as a blood grouping reagent but without the specific blood group antibody reactivity. If the reagent control contains serum or plasma, the reagent control should be shown to be free from specific blood group antibody reactivity. Standard operating procedure (SOP) to prepare the Reagent Solution for chemical analysis in the quality control laboratory. General Reagent Solution Preparation 1.0 Objective. To lay down the procedure for the preparation of the general reagent solution. 2.0 Scope1. Control cultures: For each order of Quanti Trays with different lot numbers, analytical procedures are checked by testing with known positive and negative control cultures. For example, E. coli is a positive control for this analysis and Staphylococcus aureus is a .
URINE DIPSTICK Multistix® 10SG Operator Competency
URI MLS: Urinalysis Ch. 5 (Chemical Examination) Flashcards
log rank test r package
100 Pandas is an online video slot game developed by IGT that is based on Chinese pandas in their natural habitat. This game is full of features including stacked wilds, scatters, and a free spin bonus. 100 Pandas is available on both Mac and mobile devices, as well as via an app that can be downloaded for . Ver mais
by-bottle quality control reagent testing|URI MLS: Urinalysis Ch. 5 (Chemical Examination) Flashcards